Our Innovation Journey
Glenmark’s pursuit of innovative drug discovery started over two decades ago with a vision to find cures for the unmet medical needs of patients across the globe by providing novel therapeutic options.
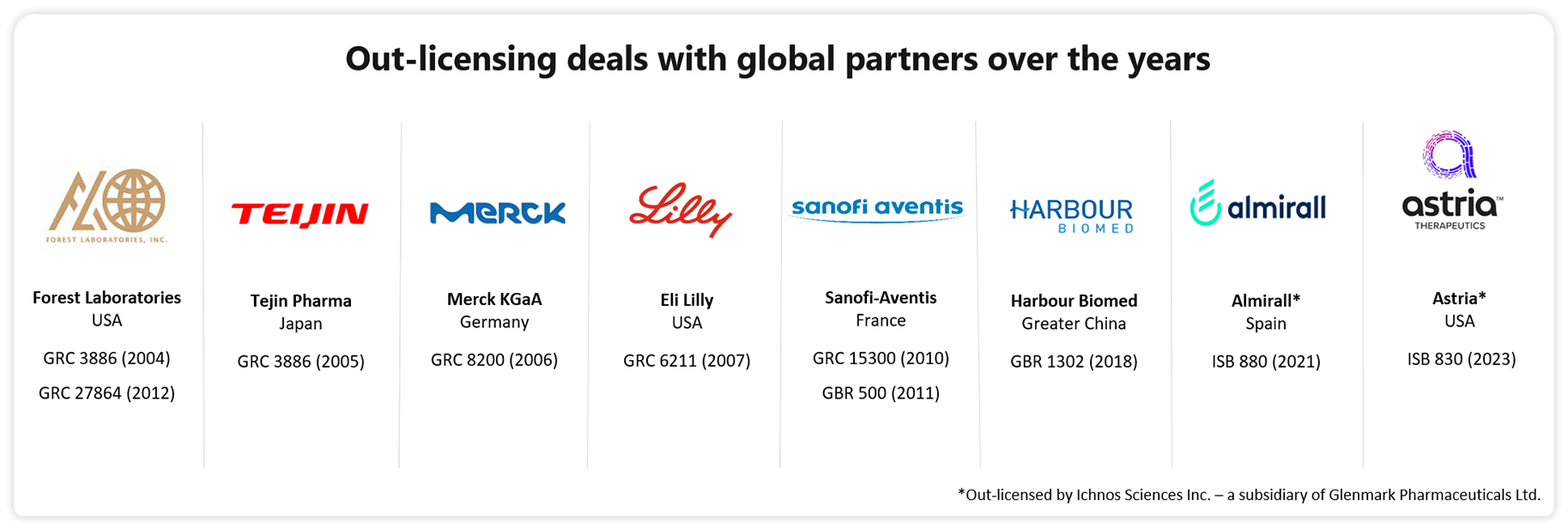
Glenmark set up its first center focusing on small molecule research at Mahape, India in 2000, followed by a biologics research center in Switzerland in 2006. One of the pivotal moments in Glenmark’s innovation journey came in 2004 when it signed its first out-licensing deal with Forest Laboratories. Subsequently, over the years, Glenmark has struck multiple out-licensing deals with large multinationals for its small and biologics molecules in various stages of clinical development.
This marked a major step for Glenmark as it continued its drug discovery journey with a primary focus on expanding its oncology and immunotherapy pipeline.
In October 2019, Glenmark took the significant step of spinning its innovative biologics research division into a wholly-owned subsidiary, Ichnos Sciences Inc., an independent company headquartered in the USA, with a heightened focus on accelerating novel biologics entities (NBE) research.
To further explore groundbreaking solutions in the treatment of cancer, Glenmark and Ichnos took a collaborative leap in January 2024 and formed
‘Ichnos Glenmark Innovation’ (IGI). This alliance combines Glenmark’s research and development proficiencies in small molecule research with those of Ichnos in NBEs to develop cutting-edge therapy solutions that treat hematological cancers and solid tumors. IGI will use different modalities to treat cancer by going after bispecific and multispecific antibodies and small molecules. Harnessing the combined proficiency of over 150 scientists, IGI will leverage the capabilities of all its global centers of innovation. Going forward, all of Glenmark’s efforts in innovation will be channelized through IGI.
For further information on IGI, click here
Out-licensing deals over the years

Glenmark’s 100% subsidiary Ichnos Sciences and Almirall enter into a licensing agreement for first-in-class iL-1RAP antagonist monoclonal antibody ISB 880. Under the agreement, Almirall is granted global rights to develop and commercialize this monoclonal antibody for autoimmune diseases. Ichnos will retain rights for antibodies acting on the IL-1RAP pathway for oncology indications.

Glenmark out-licenses mPGES-1 inhibitor to Forest Labs for USD 15 Mn (upfront payment)

Glenmark out-licenses GBR 500 to Sanofi-Aventis for USD 50 Mn (upfront payment) and USD 5 Mn (milestone payment) in 2014

Glenmark out-licenses GRC 15300, a first-in-class TRPV3 antagonist to Sanofi-Aventis for USD 25 Mn (upfront payment)
Glenmark out-licenses TRPV1 antagonist molecules to Eli Lilly for USD 45 Mn (upfront fee)

Glenmark out-licenses Melogliptin to Merck KGaA for USD 31 Mn (total payment)

Glenmark out-licenses Oglemilast to Teijin Pharma, Japan for USD 6 Mn (upfront payment)

Glenmark out-licenses GRC 3886 to Forest Laboratories for USD 35 Mn (upfront and milestone payments)
